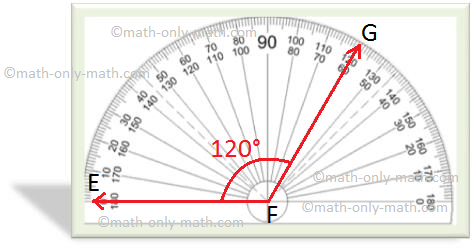
Covalent lewis dot compounds structures bond f2 line single electron ppt powerpoint presentation bonding represented
Table of Contents
Table of Contents
Have you ever struggled with drawing covalent bonds lewis structure? Perhaps you’ve found yourself staring at a blank sheet of paper, unsure of where to start or how to properly represent the bonds between atoms. Fear not, as drawing covalent bonds lewis structure is a fundamental skill in chemistry that is easy to learn with a little bit of guidance.
Many students find drawing covalent bonds lewis structure difficult due to its abstract nature. It can be challenging to visualize how the electrons are shared between atoms and how to accurately depict the resulting compound. Additionally, incorrect drawings can lead to confusion and mistakes when solving complex chemistry problems.
To draw a covalent bonds lewis structure, you must first understand the concept of valence electrons. These are the outermost electrons of an atom and are involved in the formation of chemical bonds. The key to drawing a covalent bonds lewis structure is to determine how many valence electrons each atom has and how they will be shared between the atoms involved.
In summary, to draw a covalent bonds lewis structure, you must follow these steps: determine the number of valence electrons each atom has, determine the number of bonds needed to complete the octet rule, place the atoms in the correct spatial orientation, and finally, draw the electrons shared between the atoms.
My Personal Experience with Drawing Covalent Bonds Lewis Structure
I remember struggling with drawing covalent bonds lewis structure when I first started studying chemistry. However, with practice and guidance from my teacher, I was able to develop a strong understanding of the concept. One strategy that helped me was to always start by identifying the number of valence electrons each atom had, as this laid the foundation for the rest of the drawing.
Another helpful tip was to pay close attention to how the atoms were arranged spatially. This helped me to visualize how the electrons were shared and to accurately represent the final compound. With these strategies, I was able to master drawing covalent bonds lewis structure and excel in my chemistry courses.
Common Mistakes in Drawing Covalent Bonds Lewis Structure
Some common mistakes that students make when drawing covalent bonds lewis structure include forgetting to place lone pairs of electrons, drawing incorrect spatial orientation of atoms, and failing to account for the number of electrons needed to complete the octet rule. These mistakes can lead to confusion and incorrect results when solving chemistry problems. To avoid these mistakes, it is important to carefully follow the steps outlined above and to double-check your work for accuracy.
The Octet Rule and Drawing Covalent Bonds Lewis Structure
The octet rule is a fundamental concept in chemistry that states that atoms tend to combine in a way that allows them to have eight valence electrons. This is accomplished by sharing electrons in a covalent bond with another atom or by transferring electrons in an ionic bond. When drawing covalent bonds lewis structure, it is essential to consider the octet rule and to ensure that all of the atoms have eight valence electrons.
Tips for Drawing Covalent Bonds Lewis Structure
Some additional tips for drawing covalent bonds lewis structure include starting with the atom that has the fewest electrons, using dashes to represent shared electrons, and placing lone pairs of electrons where necessary. It is also important to double-check your work for accuracy and to practice regularly to improve your skills.
Question and Answer Section
Q: What is a covalent bond?
A: A covalent bond is a type of chemical bond that involves the sharing of electrons between atoms.
Q: How many valence electrons does carbon have?
A: Carbon has four valence electrons.
Q: What is the octet rule?
A: The octet rule is a fundamental concept in chemistry that states that atoms tend to combine in a way that allows them to have eight valence electrons.
Q: What is the purpose of drawing covalent bonds lewis structure?
A: Drawing covalent bonds lewis structure is a tool used to represent the sharing of electrons between atoms in a covalent bond. It helps to visualize the structure and properties of the molecule and aids in solving chemistry problems.
Conclusion of How to Draw Covalent Bonds Lewis Structure
In conclusion, drawing covalent bonds lewis structure is a fundamental skill in chemistry that is essential for success in the field. By following the steps outlined above and practicing regularly, you can master this concept and excel in your studies. Remember to consider the octet rule, pay close attention to spatial orientation, and double-check your work for accuracy. With these tips, you’ll be a pro at drawing covalent bonds lewis structure in no time!
Gallery
The Covalent Bond | CK-12 Foundation

Photo Credit by: bing.com / lewis structure diagram molecule electron bond covalent molecular oxygen bonds double orbital shown dots below atoms between two electrons align
PPT - Lewis Dot Structures Of Covalent Compounds PowerPoint
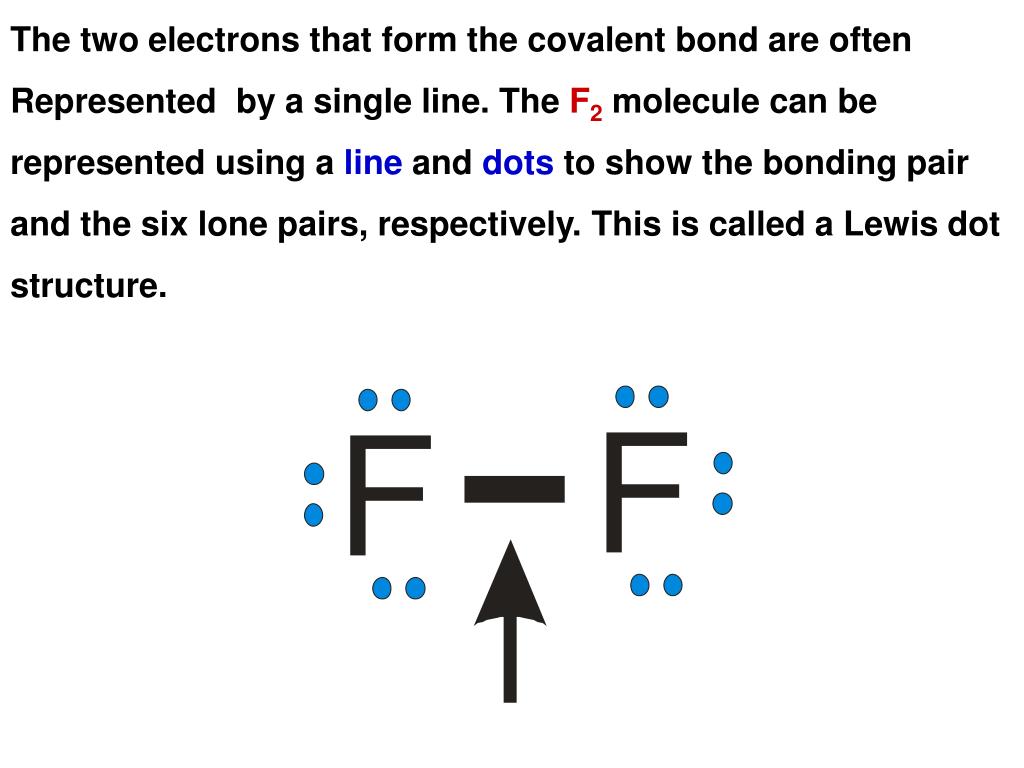
Photo Credit by: bing.com / covalent lewis dot compounds structures bond f2 line single electron ppt powerpoint presentation bonding represented
Chem Is Na Hard: September 2013
Photo Credit by: bing.com / lewis covalent structures compounds bond bonds electron formula bonding dot many diagram ionic lines example chemistry pairs there diagrams double
Question #4fc53 + Example
Photo Credit by: bing.com / covalent kovalen ikatan ionic senyawa nonpolar compounds chemische bindung h2o kimia coordinate perhatikan pcl3 foundation pcl tutor
Lewis Theory Of Bonding - Chemwiki
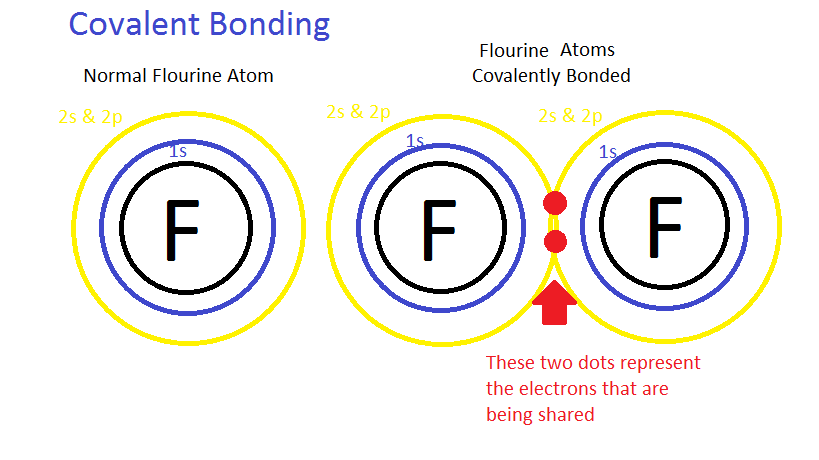
Photo Credit by: bing.com / bonding bonds chemical covalent lewis bond atoms draw dot electrons structure electron chemistry two form together molecules theory ionic compounds