Electron configurations orbitals sublevel each its chemistry orbital box own line these most library
Table of Contents
Table of Contents
Are you struggling to understand how to draw electron configuration orbitals? Do the terms “Aufbau diagram” and “orbital notation” leave you scratching your head? Fear not, for by the end of this article, you will have a clear understanding of how to draw electron configuration orbitals.
Understanding electron configuration can be a challenge for many students studying chemistry. However, it is an essential concept that lays the foundation for understanding chemical bonding and reactivity. The process of drawing electron configuration orbitals can seem daunting and confusing, but with a little practice, it can become second nature.
The first step to drawing electron configuration orbitals is to determine the number of electrons in the atom. This information can be found on the periodic table, where each element is listed with its atomic number, which represents the number of protons in the nucleus of the atom.
Once you have the number of electrons, the next step is to determine the electron configuration by filling the orbitals in order of increasing energy. The first orbital to fill is the 1s orbital, followed by the 2s, then the 2p, and so on. Each orbital can hold a maximum of two electrons with opposite spins.
My Personal Experience with Drawing Electron Configuration Orbitals
When I first started learning about electron configuration, I felt overwhelmed and confused. I didn’t understand the difference between the different types of orbitals or how to determine the order of filling. However, with the help of my teacher and some practice, I was able to master the concept and even found it enjoyable. Drawing electron configuration orbitals became like solving a puzzle, and I enjoyed the challenge of filling the orbitals in the correct order.
The Importance of Drawing Electron Configuration Orbitals
Understanding how to draw electron configuration orbitals is essential for predicting the behavior of atoms in chemical reactions. By knowing the electron configuration of an atom, we can determine its valence electrons, which are the electrons involved in chemical bonding. Additionally, understanding electron configuration can help us understand why certain elements have similar chemical and physical properties.
Filling Orbitals Using the Aufbau Principle
The Aufbau principle states that electrons fill orbitals in order of increasing energy, starting with the lowest energy orbital. This means that the 1s orbital will fill before the 2s orbital, and the 2s orbital will fill before the 2p orbitals. The order in which the 2p orbitals fill is slightly different, with the 2px orbital filling before the 2py and 2pz orbitals.
Orbital Notation vs. Electron Configuration
There are two methods to represent electron configurations: orbital notation and electron configuration notation. Orbital notation uses arrows pointing up or down to represent electrons in each orbital, while electron configuration notation uses the symbol of the occupied sublevel enclosed in brackets, followed by the number of electrons in that sublevel as a superscript. For example, the electron configuration of carbon can be written as 1s22s22p2 in electron configuration notation and ↑↓ ↑↓ ↑ ↑ in orbital notation.
Practice Makes Perfect
The process of drawing electron configuration orbitals may seem overwhelming at first, but with practice, it can become second nature. Start by mastering the first few elements and gradually work your way up to more complex elements. You may find it helpful to use colored pencils or pens to keep track of the different orbitals and electrons. Remember, drawing electron configuration orbitals is like solving a puzzle, and with practice, you can become a master puzzle solver!
Question and Answer
Q: How do you determine the number of electrons in an atom?
A: The number of electrons in an atom is equal to the atomic number, which represents the number of protons in the nucleus of the atom.
Q: What is the difference between electron configuration notation and orbital notation?
A: Electron configuration notation uses the symbol of the occupied sublevel enclosed in brackets, followed by the number of electrons in that sublevel as a superscript. Orbital notation uses arrows pointing up or down to represent electrons in each orbital.
Q: What is the Aufbau principle?
A: The Aufbau principle states that electrons fill orbitals in order of increasing energy, starting with the lowest energy orbital.
Q: Why is understanding electron configuration important in chemistry?
A: Understanding electron configuration is important in chemistry because it allows us to predict the behavior of atoms in chemical reactions and understand why certain elements have similar chemical and physical properties.
Conclusion of How to Draw Electron Configuration Orbitals
Drawing electron configuration orbitals can be a challenging concept to learn, but with practice, it can become second nature. Understanding electron configuration is essential for predicting the behavior of atoms in chemical reactions and can help us understand why certain elements have similar chemical and physical properties. By mastering the process of drawing electron configuration orbitals, you can become a master puzzle solver and ace your chemistry exams!
Gallery
Electron Configurations | CK-12 Foundation

Photo Credit by: bing.com / electron configurations orbitals sublevel each its chemistry orbital box own line these most library
Orbital Diagrams And Electron Configuration - Basic Introduction

Photo Credit by: bing.com / orbital electron configuration practice diagrams problems chemistry
Why Are Orbitals Described As Probability Maps? | Socratic
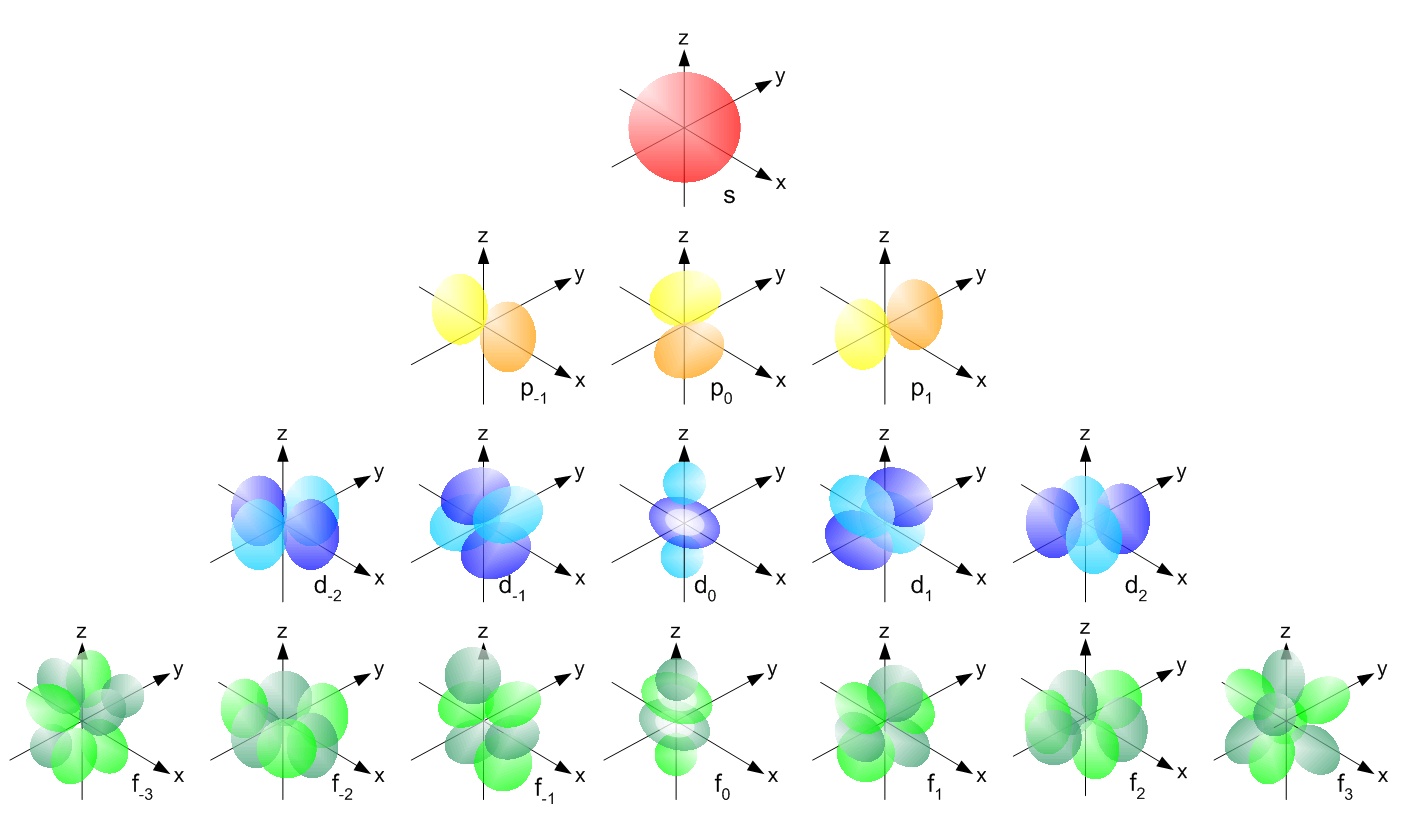
Photo Credit by: bing.com / orbitals probability orbital electron shapes single described diagram maps why atomic atom quantum orbitales electronic these structure radial electrons graphs
Aluminum Electron Configuration - YouTube
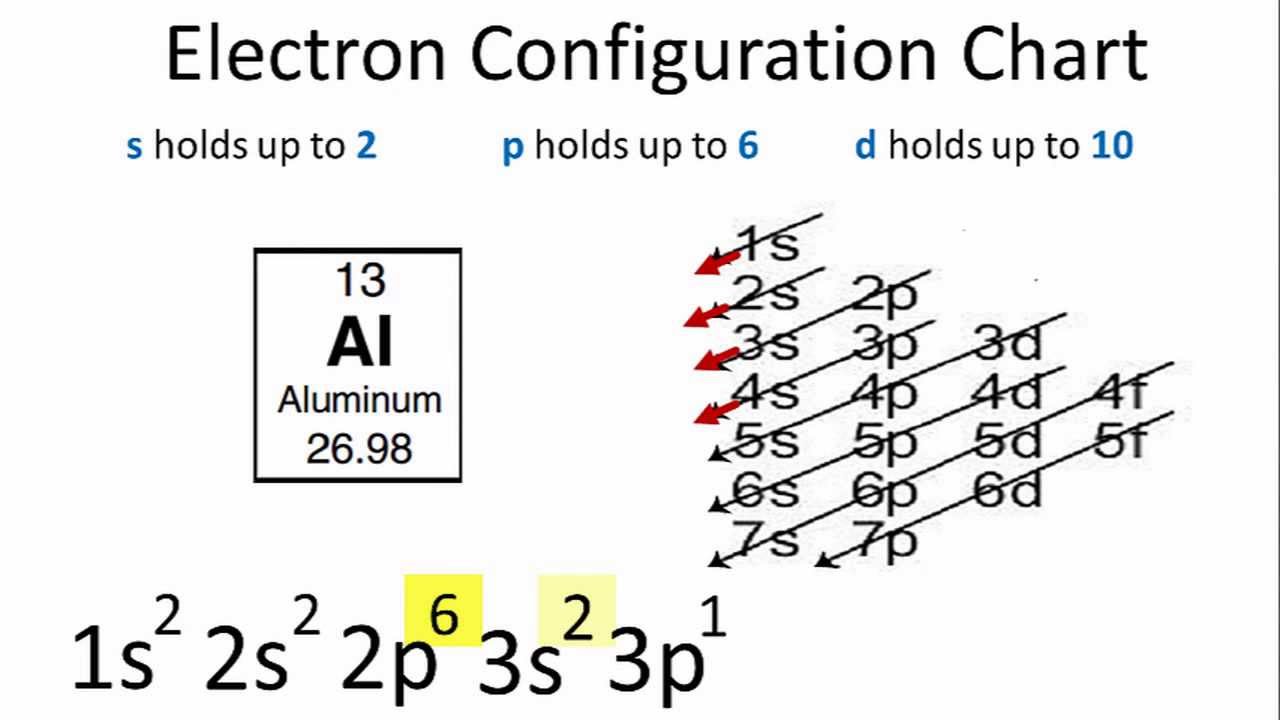
Photo Credit by: bing.com / aluminum configuration electron
Drawing Electron Configurations With Aufbau/orbital Diagram - YouTube
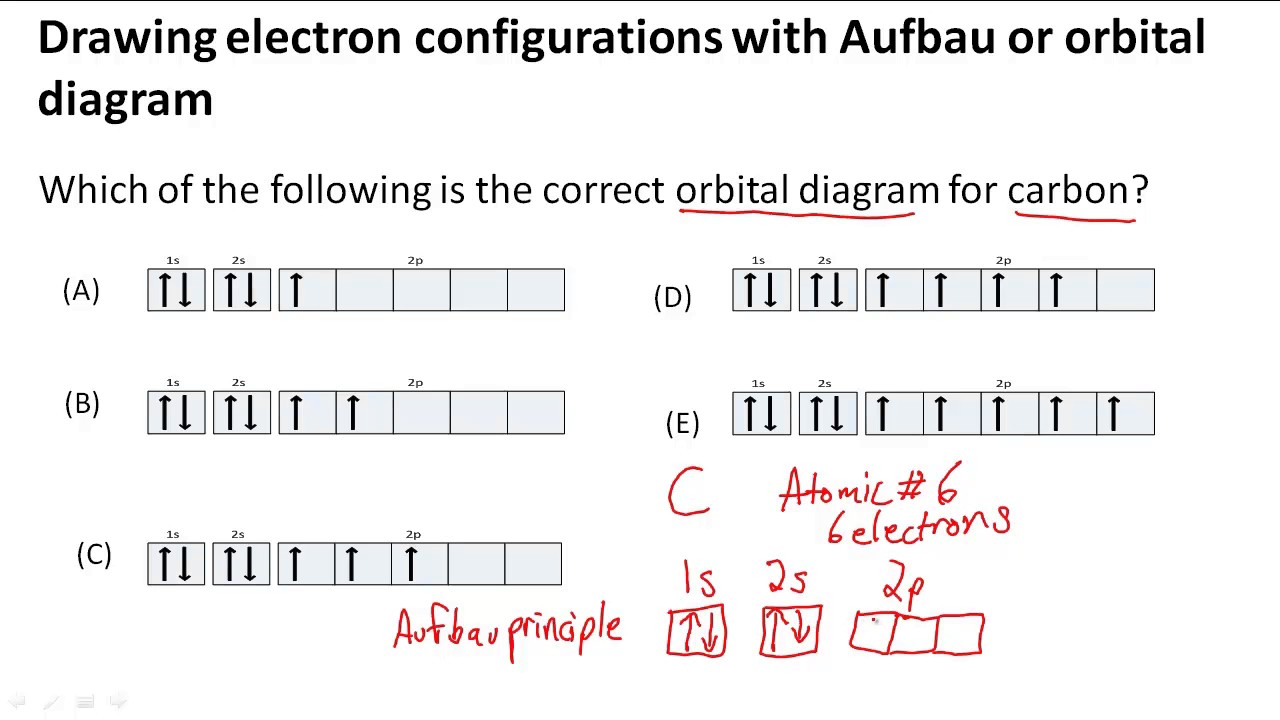
Photo Credit by: bing.com / orbital diagram electron configuration scandium aufbau drawing diagrams example arrangement configurations atoms rules