Drawing atoms montessori muddle
Table of Contents
Table of Contents
Are you struggling to figure out how to draw electrons? Drawing electrons in an atom is essential to understanding the basic principles of chemistry. Whether you are studying for school or just have a general interest in learning about atoms, this post will guide you through how to draw electrons and related keywords.
Understanding Pain Points Related to How to Draw Electrons
One of the primary pain points for students when it comes to drawing electrons is understanding the basic principles of the atom. Without this foundational knowledge, it can be challenging to figure out which electrons to draw and where to place them. Additionally, figuring out the number of electrons can be confusing, as it depends on the atomic number of the element.
Answering the Target of How to Draw Electrons
The key to drawing electrons is understanding periodic table trends and the basic structure of the atom. First, find the atomic number of the element you want to draw. Then, use the periodic table to determine the electron configuration of the element. Finally, place the electrons in the atom’s shells, beginning with the innermost shell and filling up to the outermost shell in a way that follows certain rules.
Summarizing the Main Points of Drawing Electrons and Related Keywords
In summary, understanding basic principles and periodic table trends is crucial to figuring out how to draw electrons. Make sure to start with the atomic number, use the periodic table to determine the electronic configuration, and follow the rules of filling the shells from the innermost to the outermost. Doing so will help ensure that you draw an accurate diagram of the atom with its electrons.
The Target of How to Draw Electrons and a Personal Experience
When I first started learning about how to draw electrons, I struggled with understanding the periodic table trends and determining the atomic number. However, with practice and guidance from my teacher, I was eventually able to understand and apply the rules for placing electrons in atom shells. The key is to take it step by step and not get discouraged if you make mistakes along the way.
One useful tactic to remember is to use the periodic table as a reference tool. It shows the element’s atomic number, which you can use to determine how many electrons should be in the atom’s shells. From there, it’s just a matter of placing the electrons according to certain rules.
Understanding Ionization Energy and the Relationship to Drawing Electrons
Ionization energy is the energy required to remove an electron from an atom. The ionization energy of an atom can be used to find the number of valence electrons, which are the electrons in the outermost shell of an atom. This is crucial to determining how to draw electrons, as the valence electrons are the ones that participate in chemical reactions.
Diving Deeper into Drawing Electrons and Related Keywords
When drawing electrons, it’s essential to understand the concept of electron shells. These shells are the areas of the atom where the electrons are located. The shells have different energy levels and can only hold a specific number of electrons. The first shell can hold up to two electrons, while the second shell can hold up to eight. The third shell can hold up to 18, and so on.
Understanding Sublevels and How They Impact Electron Placement
The sublevel describes the shape of the region of the atom where the electron is most likely to be found. There are four sublevels: s, p, d, and f. The s sublevel has one spherical shape and holds up to 2 electrons. The p sublevel has three dumbbell-shaped orbitals and holds up to 6 electrons. The d sublevel has five different shaped orbitals and holds up to 10 electrons. The f sublevel has seven uniquely shaped orbitals and holds up to 14 electrons.
A Personal Experience with Drawing Electrons
One practical strategy that helped me when learning how to draw electrons was practicing different elements and their electron configurations. I started with the first few elements to allow myself to gain familiarity with the periodic table trends and the different electronic configurations. Eventually, I found that the more I practiced, the easier it became, and drawing electrons became second nature to me.
Question and Answer Section
1. How can I determine an atom’s valence electrons?
An atom’s valence electrons can be found by looking at the group number (the vertical column) in which the atom is located in the periodic table of elements.
2. Can all the electrons in the atom move from one shell to another?
No, not all electrons can move between shells. Electrons can only move between shells if they have enough energy to do so.
3. Can an electron be in two shells simultaneously?
No, an electron can only occupy one shell at a time.
4. Why are the electrons negatively charged?
Electrons have a negative charge because they are made up of negatively charged particles called electrons, protons, and neutrons. The electrons have a negative charge, while the protons have a positive charge, and the neutrons are neutral.
Conclusion of How to Draw Electrons
Now that you have a better understanding of how to draw electrons, take some time to practice using the periodic table and remembering the fundamental principles of the atom. With persistence and effort, you too can master the art of drawing electrons in no time.
Gallery
Chemistry – Make Science Easy
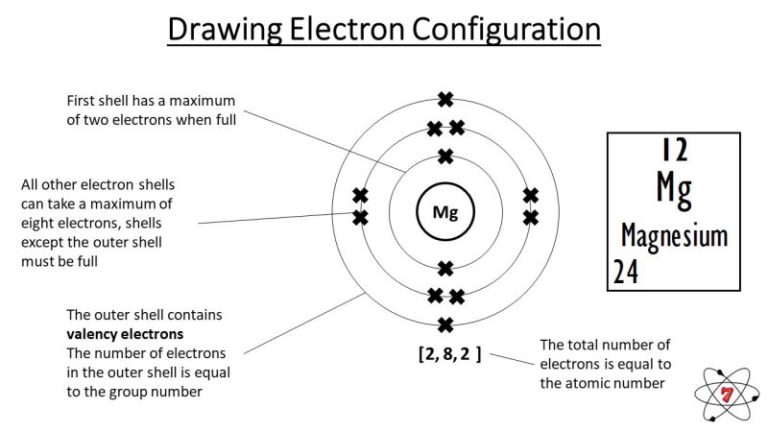
Photo Credit by: bing.com / chemistry configuration electron draw easy previous science
Atoms And Behavior – StevensBx ABA Blog

Photo Credit by: bing.com / valence electrons electron atom elektron valensi nucleus atoms inti contoh behavior serta scienceabc menentukan jumlah proton kimia bonding bohr neutron
Drawing Electron Configurations - YouTube
Photo Credit by: bing.com / electron drawing
Drawing Atoms – Montessori Muddle

Photo Credit by: bing.com / oxygen atom drawing atoms diagram shells
How To Draw Electron Shells. Basic Of Atom Part 3. GCSE Chemistry - YouTube

Photo Credit by: bing.com / draw electron shells gcse





